Patients who don’t take their medications or take them other than as prescribed often put themselves at risk for problems including misdiagnoses, complications, and death. The resulting costs to the U.S. health care system have been estimated at $105 billion annually. In clinical trials, patient nonadherence to medication regimens can lead to inaccurate and misleading results, potentially delaying the introduction of effective new treatments. To prevent such scenarios, clinicians and researchers need an inexpensive, reliable, and safe method of monitoring whether patients are taking their medications as directed.
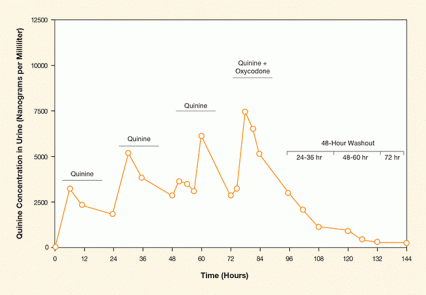 Figure. Quinine Pharmacokinetics Quinine concentrations in urine were relatively consistent during a 4-day administration period and remained detectable for more than 40 hours after the last quinine dose. Oxycodone taken 2 hours after quinine did not affect quinine detection, nor did quinine affect oxycodone concentrations.
Figure. Quinine Pharmacokinetics Quinine concentrations in urine were relatively consistent during a 4-day administration period and remained detectable for more than 40 hours after the last quinine dose. Oxycodone taken 2 hours after quinine did not affect quinine detection, nor did quinine affect oxycodone concentrations.
- Text Description of Graphic
-
The figure shows a line graph of quinine concentrations in urine over a 144-hour dosing period. The vertical (y-) axis shows quinine concentration in urine in nanograms per milliliter, and the horizontal (x-) axis shows the time in hours. Times of quinine administration (at about 6, 30, and 55 hours) and quinine plus oxycodone administration (at about 76 hours) are indicated by horizontal lines, and a 48-hour washout period of no quinine administration from 96 to 144 hours is indicated by a bracketed line divided into 24 to 36 hours, 48 to 60 hours, and 72 hours. After the first administration, quinine concentration reached a peak of about 3,000 nanograms per milliliter at about 6 hours, followed by a decline to about 2,400 nanograms per milliliter at 24 hours; after the second administration, quinine rose to about 5,000 nanograms per milliliter at about 32 hours, followed by a decline to about 2,600 nanograms per milliliter at about 58 hours; following the third administration, quinine rose to about 6,000 nanograms per milliliter at about 60 hours and declined to about 2,600 nanograms per milliliter at about 74 hours; and after the fourth administration along with oxycodone, quinine rose to 7,500 nanograms per milliliter at about 76 hours and declined to about 2,600 nanograms per milliliter at 96 hours. During the 48-hour washout period from 96 to 144 hours, the quinine concentration gradually dropped to just above the detection threshold.
Dr. Shanna Babalonis and colleagues at the University of Kentucky suggest that adding low doses of quinine to patients’ medications could serve the purpose. The researchers gave nine volunteers once-daily doses of 80 milligrams of quinine for 4 days. Quinine concentrations, measured in the volunteers’ urine, remained relatively steady during quinine dosing, then decreased in the 3 days after quinine dosing was stopped (see Figure). Because quinine has a rather predictable rate of elimination, clinicians or researchers could potentially ascertain whether a patient had taken it—and the medication with which it is combined—as directed.
One class of medications that poses particular risks when used other than as prescribed is the opioids. To test whether quinine might be used specifically to monitor adherence to opioid regimens or in clinical trials enrolling opioid users, the researchers gave the volunteers, on different study days, oxycodone alone, quinine alone, and quinine combined with oxycodone. (All the volunteers were opioid users who were not addicted and not seeking treatment.) Neither the quinine nor the oxycodone behaved significantly differently when the two were given together. The finding suggests that quinine has the potential to be a safe and reliable tracer for opioid adherence or in clinical trials testing new medications for opioid use.
Although quinine produces adverse effects when used to treat malaria, the study volunteers reported no problems related to the much smaller dosage used in this study.
The researchers caution that additional studies are needed to determine whether low doses of quinine interact with other common prescription medications or with illicit drugs. Such studies could also evaluate whether quinine is a reliable marker for medication adherence in larger and more diverse patient populations.
This study was supported by NIH grant DA016718.
Source:
Babalonis, S.; Hampson, A.J.; Lofwall, M.R., et al. Quinine as a potential tracer for medication adherence: A pharmacokinetic and pharmacodynamic assessment of quinine alone and in combination with oxycodone in humans. The Journal of Clinical Pharmacology 55(12):1332-1343, 2015. Abstract