NIDA-supported researchers have shown that giving mice a modified version of a naturally occurring gene blocks cocaine’s stimulant effects without affecting the animals’ physiological or metabolic health. The new evidence advances the proposed therapy a step closer to readiness for testing in people.
Drs. Vishakantha Murthy and Stephen Brimijoin and colleagues at the Mayo Clinic and the Ottawa Hospital Research Institute, together with Dr. Chang-Guo Zhan at the University of Kentucky, have engineered a modified version of a gene that occurs naturally in the body. The natural gene produces an enzyme, called butyrylcholinesterase (BChE), which splits the cocaine molecule into two harmless compounds. The modified gene produces a variant of BChE, called cocaine hydrolase (CocH), that destroys cocaine thousands of times more effectively than BChE. As a result, CocH promptly neutralizes doses of cocaine that overwhelm the capacity of the naturally occurring enzyme.
If the gene therapy advances to clinical use, it will be given to patients to help them avoid relapse to cocaine use. If a patient should slip and take cocaine, the enzyme will intercept and cleave the drug before it reaches the brain. In this way, the BChE enzyme will prevent euphoria, reduce motivation for subsequent cocaine use, and shield the patient from the cocaine’s toxic and addictive effects.
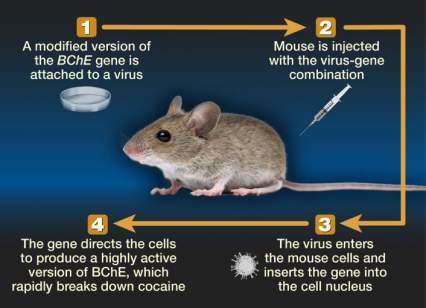
To transfer the CocH gene to patients, the researchers have attached it to a virus that is stripped of its ability to reproduce or cause disease (see Figure 1). The virus transports the modified gene into liver cells, primarily, and stimulates their protein-building machinery to produce CocH.
- Text description of Figure 1
-
The figure shows a schematic illustration of the gene therapy approach to treat cocaine addiction with the BChE gene in a mouse model. Step 1 shows a petri dish, representing a laboratory procedure in which the BChE gene is attached to a virus; in step 2, the virus-gene combination is injected into a mouse; in step 3, the injected virus-gene combination enters the mouse’s cells and is inserted into the cell nucleus; and in step 4, the virus-gene combination directs the mouse cells to produce a highly active version of the BChE enzyme, which rapidly breaks down cocaine.
In the recent study, consistent with previous findings, mice with the transplanted CocH gene developed enzyme levels in their blood that were 1,500-fold higher than those produced in control animals by the BChE gene. The animals with the transplanted gene broke down cocaine up to 500,000 times faster than control animals.
When the researchers administered cocaine to the mice carrying the CocH gene, the animals exhibited no increase in activity or other sign of the drug’s stimulant effects (see Figure 2). Even a dose of cocaine that is normally lethal (100 mg/kg body weight) did not elicit any of the responses, such as increased running activity, that animals normally exhibit after exposure to the drug. Blood levels of CocH remained high and the enzyme remained active for over 16 months.
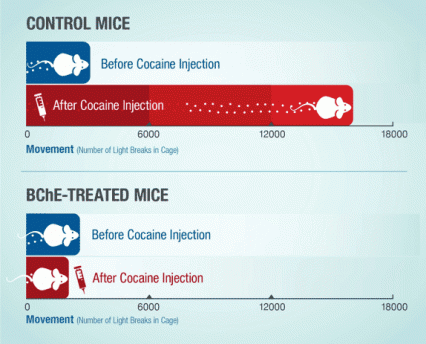 Figure 2. Modified BChE Enzyme (CocH) Suppresses Cocaine-Induced Locomotor Stimulation Mice with the modified BChE (CocH) gene did not move about their chambers more after receiving and injection of cocaine, in contrast to control mice whose movement increased many-fold. The graph shows how many times each group of animals crossed a light beam in their cage, on average, during the 3-hour period before cocaine injection, and the 3-hour period after injection.
Figure 2. Modified BChE Enzyme (CocH) Suppresses Cocaine-Induced Locomotor Stimulation Mice with the modified BChE (CocH) gene did not move about their chambers more after receiving and injection of cocaine, in contrast to control mice whose movement increased many-fold. The graph shows how many times each group of animals crossed a light beam in their cage, on average, during the 3-hour period before cocaine injection, and the 3-hour period after injection.
- Text description of Figure 2
-
The figure shows an infographic with two stacked, horizontal bar charts indicating the effect of cocaine on running activity in control mice and mice injected with the modified BChE gene. The horizontal axis indicates the mice’s running activity as the number of light breaks in a cage, and the vertical axis mice before and after cocaine injection. As shown in the top chart, a single cocaine injection strongly increases running activity in the control mice from less than 3,000 light breaks before the injection to about 16,000 breaks after the cocaine injection. As shown in the bottom chart, injection with the modified BChE gene construct abolishes the effect of the cocaine injection on running activity of the mice, indicated by similar numbers of light breaks, i.e., of 2,000 to 3,000, before and after the mice were injected with cocaine.
The primary aim of the recent experiments was to determine if the gene transfer would alter the animals’ physiological and metabolic function. The researchers monitored the animals’ body composition, motor activity, feeding and metabolic rate, and glucose tolerance. Measurements taken when the animals had been given the CocH gene and were actively producing CocH disclosed no treatment-related or drug-related changes in any of these parameters. This was the case during the daytime and also at night, whether the animals were well fed or fasting, and after they received an injection of cocaine.
The researchers noted two alterations in mice related to the gene transfer, neither of which they judge to necessarily pose an obstacle to successful clinical use:
- Mice that were injected with a modified human BChE gene lost a modest amount of weight compared with control mice. The researchers suggest that the finding probably reflects a low-level immune response mounted by the animals against a gene from a different species, because mice that were given a modified mouse BChE gene showed no such effect. In clinical use, of course, patients would receive a modified human gene.
- Because BChE enzyme can break down the neurotransmitter acetylcholine, the researchers tested whether mice that received the gene for the more highly active CocH enzyme might experience impaired muscle strength, motor activity, or cognition. However, the mice with the transplanted gene showed no decrements in any such functions, including grip strength, exercise endurance, or learning and memory.
Drs. Murthy and Brimijoin hope to complete the further tests required to clear their proposed gene transfer therapy for clinical trials within the next 3 years.
This study was supported by NIH grants DA023979 and DA031340.
Source:
Murthy, V.; Gao, Y.; Geng, L. et al. Physiologic and metabolic safety of butyrylcholinesterase gene therapy in mice. Vaccine 32(33):4155-4162, 2014. Full text