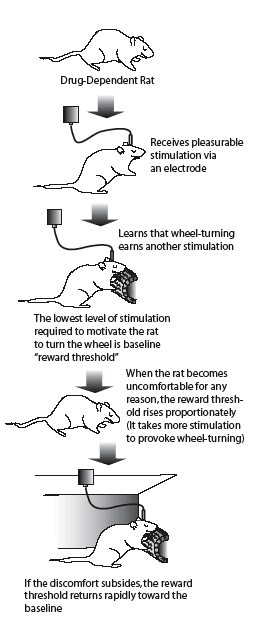
 Asking a Rat, "How Do You Feel?" Dr. Athina Markou and her colleagues used this experimental technique, known as intracranial self-stimulation, to assess animals' discomfort from nicotine withdrawal and evaluate the role of mGluII receptors in withdrawal.
Asking a Rat, "How Do You Feel?" Dr. Athina Markou and her colleagues used this experimental technique, known as intracranial self-stimulation, to assess animals' discomfort from nicotine withdrawal and evaluate the role of mGluII receptors in withdrawal.More than a third of America's 46 million adult smokers try to stop each year, but fewer than 10 percent succeed. Some relapse because they cannot tolerate the discomfort and craving associated with nicotine withdrawal. In recent animal studies, NIDA-supported scientists identified sites on some brain cells that appear to be key promoters of the negative psychological symptoms of nicotine withdrawal. The sites, called glutamate receptors, are part of the communication network that uses the neurotransmitter glutamate as a chemical messenger.
Neurobiologists have previously shown that glutamate helps produce the good feelings smoking causes. When nicotine attaches to receptors on cells in the brain's ventral tegmental area (VTA), the cells release glutamate, which in turn triggers other VTA cells to release dopamine, a neurotransmitter that produces pleasure. Dr. Athina Markou of The Scripps Research Institute (TSRI) in La Jolla, California, and colleagues reasoned that just as glutamate surges caused by nicotine give rise to smoking pleasure, glutamate depletion related to nicotine abstinence might underlie the displeasure of withdrawal. The researchers speculated that when nicotine is withdrawn after chronic use, the feedback system that restores glutamate to normal levels following surges could overshoot its mark, resulting in a glutamate dearth—and symptoms of depression and irritability.
To test this idea, Dr. Markou and Dr. Paul Kenny at TSRI, along with Dr. Fabrizio Gasparini of Novartis Institutes for Biomedical Research in Basel, Switzerland, focused on a specific group of glutamate receptors called group II metabotropic glutamate (mGluII) receptors. These inhibitory receptors are key components of the glutamate feedback system: They detect high glutamate levels and signal glutamate-producing cells to reduce their activity to bring the levels back down. Inactivating the mGluII receptors interrupts this process, leaving glutamate levels high. The researchers hypothesized that if they inactivated rats' mGluII receptors while subjecting the animals to nicotine withdrawal, the plunge in glutamate levels may be avoided, and the animals' withdrawal symptoms attenuated.
The scientists implanted tiny pumps under the skin on the backs of adult male rats. The pumps dispensed a nicotine solution that maintained high nicotine levels equivalent to those produced in a human who smokes 30 cigarettes per day. After the rats had been exposed to nicotine for 7 days, the investigators removed the pumps, depriving the animals of nicotine and thus leading to nicotine withdrawal. Then, after 18 hours of withdrawal, half the rats were injected with a chemical that blocks the action of mGluII receptors, in effect switching off the inhibitory feedback signals to the glutamate-producing cells. Over the next 72 hours the scientists evaluated the rats at regular intervals using a technique, called intracranial self-stimulation (see "Asking a Rat, 'How Do You Feel?'"), that measures withdrawal-like depression in laboratory animals. As the scientists had predicted, the rats with active mGluII receptors exhibited significant discomfort; the withdrawal discomfort rapidly dissipated in those in which mGluII receptors were turned off.
To help confirm the association between mGluII receptors and withdrawal-like symptoms, Dr. Markou's team treated nicotine-dependent rats with a compound that stimulates the same receptors. In these animals, activation of the inhibitory glutamate loop triggered discomfort comparable with that in nicotine withdrawal.
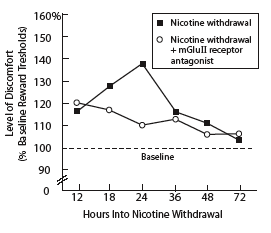 Blocking an Inhibitory Glutamate System Reduces Discomfort of Nicotine Withdrawal in Rats. Rats that had been exposed to nicotine for 7 days showed discomfort 12 hours after withdrawal from nicotine. Rats that were injected, at 18 hours into withdrawal, with a compound that blocked mGluII receptors showed no increase in withdrawal-associated discomfort. (Discomfort measurement technique is described in "Asking a Rat, 'How Do You Feel?'"). Untreated rats experienced increasing discomfort through 24 hours of withdrawal.
Blocking an Inhibitory Glutamate System Reduces Discomfort of Nicotine Withdrawal in Rats. Rats that had been exposed to nicotine for 7 days showed discomfort 12 hours after withdrawal from nicotine. Rats that were injected, at 18 hours into withdrawal, with a compound that blocked mGluII receptors showed no increase in withdrawal-associated discomfort. (Discomfort measurement technique is described in "Asking a Rat, 'How Do You Feel?'"). Untreated rats experienced increasing discomfort through 24 hours of withdrawal."Other research has shown how nicotine changes regulation of excitatory glutamate signaling," Dr. Markou says. "Our study helps explain how nicotine also commandeers inhibitory glutamate circuits. The altered function of the mGluII receptors appears to mediate, at least partly, the depressionlike aspects of nicotine withdrawal." The effect, she explains, is a carrot-and-stick influence strong enough to thwart the most sincere attempts to quit smoking. "Nicotine provides a positive effect through the excitatory circuits, making smoking a rewarding and reinforcing experience. Now we see that nicotine has a similarly powerful aversive effect through the inhibitory circuits, making withdrawal an unpleasant experience."
The role of mGluII receptors in withdrawal suggests that these receptors might also offer a target for therapeutic intervention, Dr. Markou adds. "Easing the depression like aspects of withdrawal would significantly decrease discomfort and make it easier for people to maintain abstinence and resist the temptation to relapse to smoking."
Source:
- Kenny P.J.; Gasparini, F.; and Markou, A. Group II metabotropic and alpha-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA)/kainate glutamate receptors regulate the deficit in brain reward function associated with nicotine withdrawal in rats. Journal of Pharmacology and Experimental Therapeutics 306(3):1068- 1076, 2003.
[Full Text]